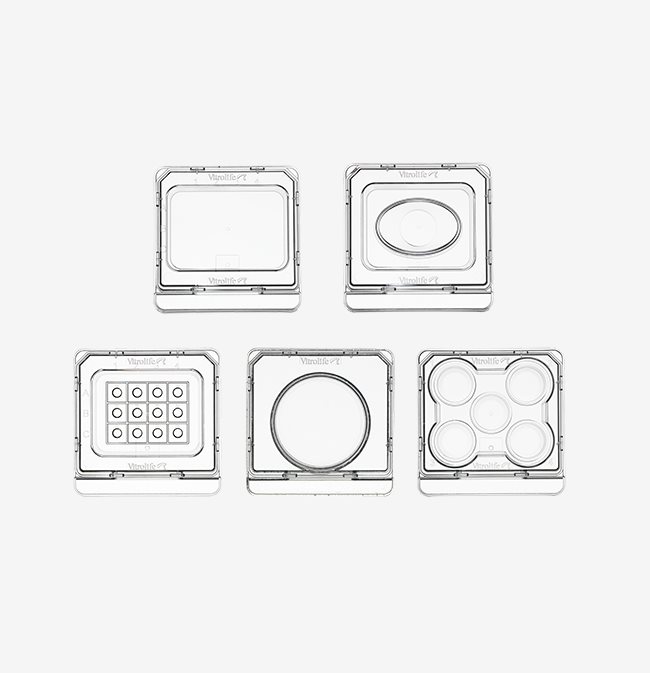
Dishes
The Vitrolife Labware dishes are specifically designed and certified for IVF, enabling a safe environment for your IVF procedures.
Application Ready to use.
Storage Store in +2 to +30°C


Challenges with plastic disposables
Optimisation of the embryo culture system
The ability to culture viable embryos involves more than using appropriate culture media. There are many variables that can have an impact on the outcome of an IVF cycle, all of which need to be taken into account in order to optimise pregnancy rates1, 2. This is particularly critical during treatment of infertility since gametes and embryos are extremely sensitive. Precautions have to be taken at every step to prevent toxic or harmful components from entering the culture system.
Plastic disposables and reprotoxicity
Plastic disposables are used throughout the IVF process, from oocyte aspiration to embryo transfer. However only a small percentage of the contact supplies and tissue cultureware used in IVF is suitably tested.
When plastic disposables are insufficiently quality controlled, they can contain components that are toxic to human reproductive cells such as gametes and embryos. This phenomenon can be referred to as reprotoxicity and is defined as a negative influence on the physiology and viability of human gametes and embryos. Reprotoxicity can result in reduced gamete and embryo viability with a subsequent reduction in implantation rate or ongoing pregnancy rates3.
Vitrolife MEA can detect sub-optimal conditions
It has been reported that not all disposables on the market used for IVF fulfil the quality standard needed for safe procedures. Approximately 25% of all contact materials failed pre-screening with an accurate and sensitive Mouse Embryo Assay (MEA) and were considered sub-optimal for IVF4.
Vitrolife has developed the most sensitive MEA protocols. These assays are capable of detecting toxic and sub-optimal raw materials, media, and contact materials. The MEA from Vitrolife is sensitive enough to identify subtle problems that will also lead to impaired human embryo development.
Learn more about how we perform MEA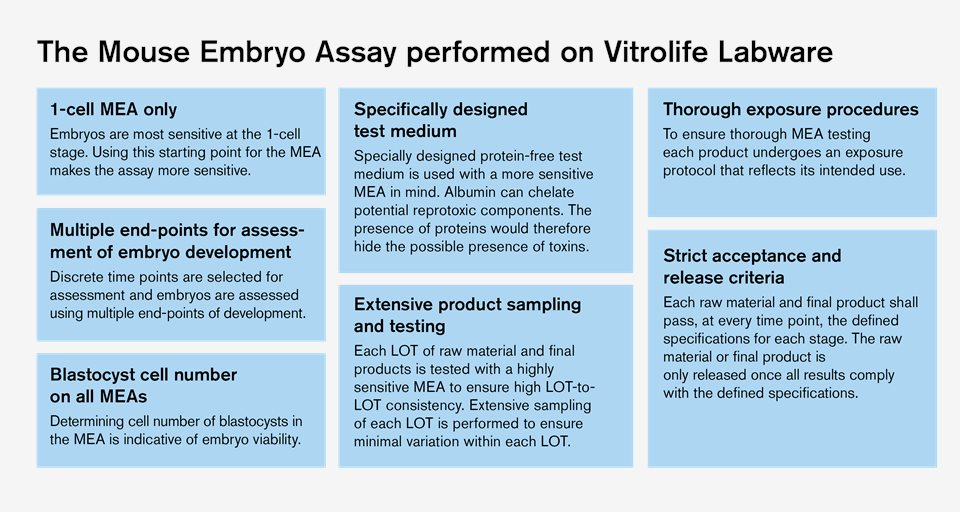
Create optimal culture conditions by understanding the importance of extensive quality testing
Plastic disposables are used throughout the IVF process, but only a small percentage of them are suitably tested. When insufficiently quality controlled, plastic disposables can contain components that are toxic to gametes and embryos.
Reprotoxicity has a negative influence on the physiology and viability of human gametes and embryos.
Reprotoxicity can however be minimised by using media and disposables that have been accurately tested.
Embryo safe dishes enabling control
Our dishes are specifically designed to support and facilitate the various procedures during the IVF process to make your way of working easier and more effective. Vitrolife dishes are manufactured with quality controlled and certified materials. The finished products are further tested to offer a secure environment for gametes and embryos.
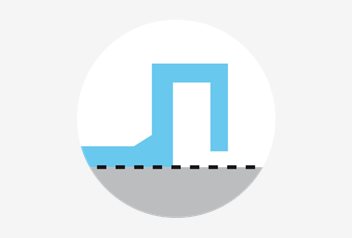
Temperature consistency
The absolute flat bottom of the square dishes, enables full contact with the heated stage. All square dishes receive the same bottom temperature when placed on a heated stage.

Labelling area
To secure patient identification, the square dishes have a dedicated area for labels or barcodes, separated from the handling area.
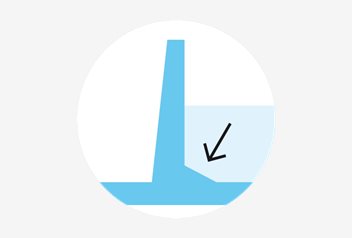
Tapered edges
Tapered edges provide easier access to embryos as they are also clearly visible at the periphery of the well. All dishes except ICSI dish and Collection dish 90 mm.
Dishes for every procedure
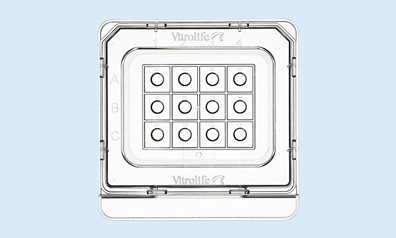
Micro-droplet Culture Dish
This unique innovation takes culture in micro-droplets to a higher level. The dish is equipped with 12 micro-wells optimised for 25-35 µL droplets. The α-numeric identification secures and facilitates embryo identification, both when viewed in and outside of the microscope.

5 Well Culture Dish
The 5 Well Culture Dish ensures embryo viability, as each well is surrounded by either air or media, resulting in a homogenous temperature.

Centre Well Dish
The Centre Well Dish is a multi-purpose dish for fertilisation, cryo-procedures, embryo culture and embryo transfer. The oval centre well allows for easy instrument access from the sides. The small diameter of the bottom facilitates easy embryo location.
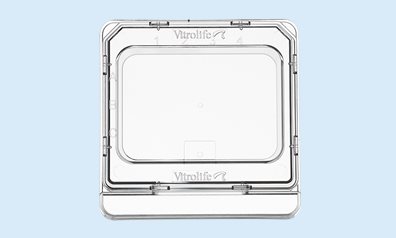
ICSI Dish
The square format and low profile with low rims allow for easy instrument access. The α-numeric identification secures and facilitates embryo identification, both when viewed in and outside of the microscope.
Culture Dish 40 mm
Like the other dishes intended for culture, this dish has tapered edges. The ramp makes for easier access to embryos as they are clearly visible when at the periphery of the wall. The dish has a security ledge.

Round Dishes 60 and 90 mm
The Collection dish 90 mm is dedicated for oocyte retrieval, while the Culture dish 60 mm can be used for oocyte retrieval, fertilisation and culture.
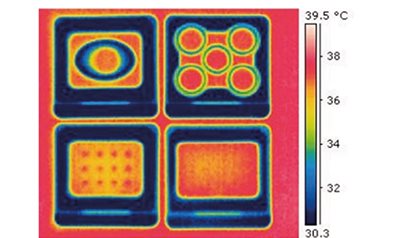
Temperature consistency ensures embryo viability
Embryos in culture are very sensitive to temperature fluctuations. The square dishes have an absolute flat bottom which enables full contact with the heated stage. The picture shows four Vitrolife Labware dishes on a heated stage calibrated to 37°C.
Once you have calibrated the temperature of your heated stage you can feel confident that all dishes will have the same temperature. This optimises heat transfer, minimises variation between dishes and enables consistent temperature.

Optimise media temperatures in dishes with VitroTemp
VitroTemp™ makes it easier to maintain the optimal temperature of media in dishes based on accurate fast and simple measurements that replicate the actual conditions for procedures used in the IVF lab. It is a specially designed temperature measurement solution that combines an advanced digital readout instrument with a custom made probe, a Pt100 sensor attached to the bottom of the Vitrolife square dishes. It’s easy to use and provides more consistent temperature measurements than most thermometers commonly used in IVF labs today1.
Learn more about VitroTemp
• Sterility assurance level (irradiation)10-6
• Virgin polystyrene
• Non-pyrogenic at less than 0.25 endotoxin units/device
• Medical Device products certified for IVF
• MEA using multiple endpoints, including 1-cell, expanded blastocyst within 96 hours ≥ 80% and cell count.
Why you should only use IVF certified plastics
There are at least 30 plastic items involved in every IVF procedure. If each of them reduce the embryo viability by 2%, the final viability at re-implantation is reduced by 44%. Download this white paper to learn about what to think about when using plastics in your IVF lab.
Download white paper

Clinical evaluation and user experiences with Vitrolife Labware dishes
The specially designed square dishes were clinically evaluated in two ways; for embryo development and clinical pregnancy and for usability and user friendliness. The results show that Vitrolife Labware dishes are safe and support treatment success when used for clinical IVF. In addition the dishes scored higher on usability compared to other dishes commonly used for IVF.
Download full summary
Maintaining the correct temperature is a critical environmental factor for gametes and embryos and needs to be carefully monitored.
- Jan Gunst, clinical embryologist, Belgium

CulturePro dish
The CulturePro dish is specially designed for group culture preference, so that 4 embryos share a 50ul droplet. Each dish has 4 rinsing wells of 100ul each and the dish uses 1.6 ml oil overlay. Convenient handling fins ensure safe transfer of dishes minimising handling risks.The CulturePro dish is designed to be used only with a CulturePro incubator.
REFERENCES 1. Gardner DK and Lane M (2007). Embryo culture systems. In Vitro Fertilization A Practical Approach. Ed. DK Gardner. Informa Healthcare, New York. pp 221-282. 2. Gardner DK (2008). Dissection of culture media for embryos: the most important and less important components and characteristics. Reprod. Fertil. Dev. 20, 9-18. 3. Nijs et al. (2009) Fertil Steril. Aug; 92(2):527-535. 4. Gardner DK (2007). Human embryonic development in vitro. In Human IVM. Ed. SL Tan. Taylor & Francis, New York. pp 295-312.
Support Documents
Documents Language

Scientific summaries
English
Clinical evaluation and user experience of Vitrolife Labware dishes
Document and Ref: Five dishes specifically developed and toxicity tested for IVF were evaluated by two clinics regarding clinical performance. The results at both clinics show that Vitrolife Labware are safe and support treatment success when used for clinical IVF. In addition, twenty clinics gave the dishes a higher score of usability than their traditional dishes showing that the new dish design is at least as user friendly as other dish types commonly used for IVF.
Do you have a question about this product?
Contact us
Find your local Vitrolife representative
Contact usMaximise success every step of the way
We provide what you need to secure improved results throughout the IVF journey.