RapidVit™ & RapidWarm™ Blast
Minimum toxic exposure. Maximum embryo support.
Description MOPS buffered media containing cryoprotectants and human serum albumin.
Purpose For vitrification and warming of blastocyst stage embryos.
Application For use in sequence after equilibration at +37°C and ambient atmosphere.
Storage Store dark at +2 to +8°C.

MOPS buffered media
RapidVit and RapidWarm Blast contains hyaluronan for optimal cryosurvival and amino acids to support embryo development. The media are buffered with MOPS to maintain stable pH during the vitrification and warming procedures and are ready to use after warming to +37°C.
Proven success
Numerous publications show that Rapid-i Vitrification System provides excellent outcomes after vitrification of all stages, from oocytes to blastocysts. Independent published studies show that Rapid-i Vitrification System performs equally well as open vitrification systems and provides safe storage. 1,2
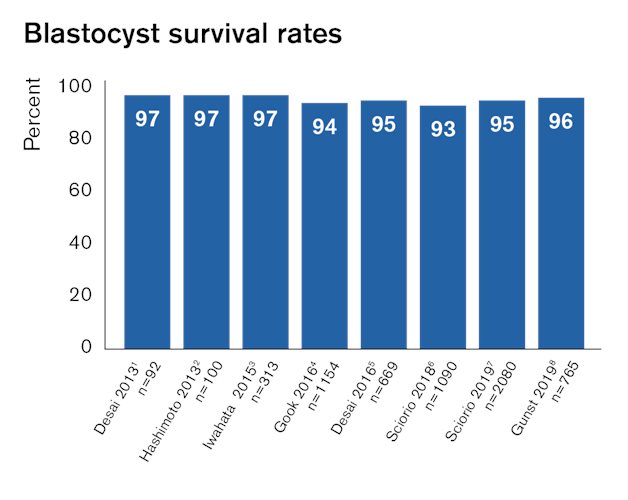
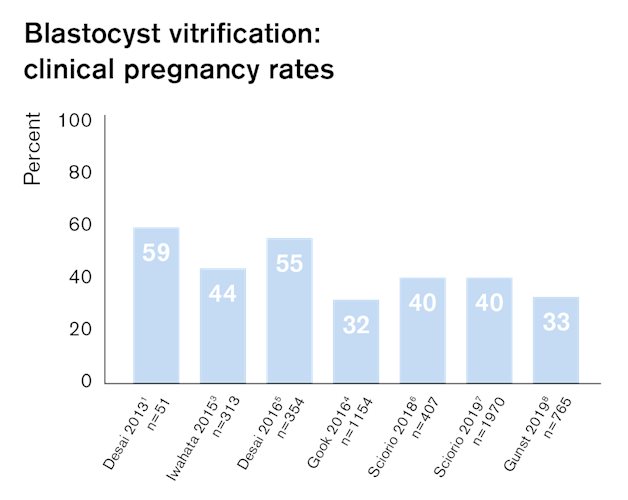
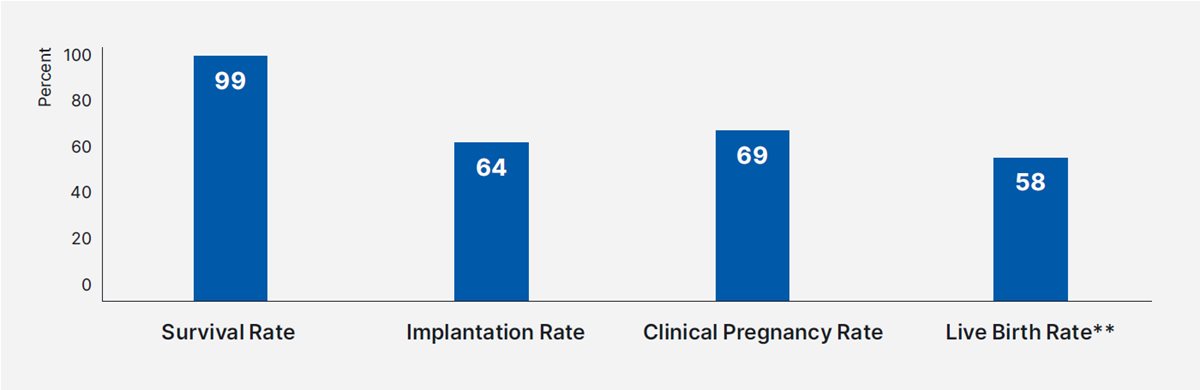
Proven effect
High survival, implantation, clinical pregnancy and live birth rates were observed for vitrified and warmed blastocysts using Rapid-i Kit, RapidVit Blast and RapidWarm Blast in a real-world clinical setting.
This real-world clinical experience data was retrospectively gathered from two clinics in the USA.
A closed device (Rapid-i™ Kit) and MOPS buffered media (RapidVit™ Blast, RapidWarm™ Blast) were used for vitrification and warming of 3 491 blastocysts. Data was collected retrospectively (Jan 2018 – Dec 2020) following 3 004 transfers from two clinics in the USA.14
Vitrification and warming were carried out at physiological temperature (37° C) which shortens the vitrification time and minimises the exposure time to cryoprotectants and their potential toxic effects.
** Live Birth Rate was provided by one clinic

Rapid-i Vitrification System is excellent for use with embryo and oocyte vitrification. Since 2009, its efficient design and security features provided an advantage in our laboratory for embryologists making handling and training simple and effective.
With the Rapid-i Vitrification System, we have high survival, clinical pregnancy and live birth rates that are reproducible and reliable from year to year.
- Sheela Ali, PhD, HCLD Scientific Director, Dallas IVF

We have been using Rapid-i Vitrification System since 2010 and we have consistently seen great survival rates, great recovery rates and ultimately great pregnancy rates.
The Rapid-i Vitrification Systems impacts our daily workflow in a positive way. It is fast, easy to use and repeatable amongst all embryologists.
- Glenn Proctor, MHA, Director of Laboratories, Conceptions Reproductive Associates of Colorado
Dr. Sheela S. Ali, a long time Vitrolife customer and Scientific Director at Dallas IVF, tells us about her experiencens with the Rapid-i™ Kit.
- Sheela Ali, PhD, HCLD Scientific Director, Dallas IVF

Warming media for every need
RapidWarm media contain decreasing concentrations of sucrose to remove membrane permeable cryoprotectants and rehydrate the cells. For more information about the sucrose levels in the Vitrolife RapidWarm media, download the product leaflet.
Download now

A complete system
Rapid-i Vitrification System is an aseptic and closed system. It offers a simple, safe and structured workflow. Rapid-i Kit is renowned for being easy to load. Oocytes are held in place by surface tension - no sticking to the device.
Learn more about Rapid-i Vitrification System
References
1. Desai NN, Goldberg JM, Austin C, Falcone T. The new Rapid-i carrier is an effective system for human embryo vitrification at both the blastocyst and cleavage stage. Reprod Biol Endocrinol. 2013 May 15;11:41.
2. Hashimoto S, Amo A, Hama S, Ohsumi K, Nakaoka Y, Morimoto Y. A closed system supports the developmental competence of human embryos after vitrification : Closed vitrification of human embryos. J Assist Reprod Genet. 2013 Mar;30(3):371-6.
3. Iwahata H, Hashimoto S, Inoue M, Inoue T, Ito K, Nakaoka Y, Suzuki N, Morimoto Y. Neonatal outcomes after the implantation of human embryos vitrified using a closed-system device. J Assist Reprod Genet. 2015 Apr;32(4):521-6.
4. Gook DA, Choo B, Bourne H, Lewis K, Edgar DH. Closed vitrification of human oocytes and blastocysts: outcomes from a series of clinical cases. J Assist Reprod Genet. 2016 Sep;33(9):1247-52.
5. Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, Falcone T. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. 2016 Nov;106(6):1370-1378.
6. Sciorio R, Thong KJ, Pickering SJ. Single blastocyst transfer (SET) and pregnancy outcome of day 5 and day 6 human blastocysts vitrified using a closed device. Cryobiology. 2018 Oct;84:40-45.
7. Sciorio R, Thong KJ, Pickering SJ. Increased pregnancy outcome after day 5 versus day 6 transfers of human vitrified-warmed blastocysts. Zygote. 2019 Oct;27(5):279-284.
8. Gunst J. et al, Data on file, 2019. Slow developing embryos undergoing compaction or cavitation on day 5 substantially contribute to live birth rates after single day 6 vitrified-warmed blastocyst transfer, P-135, 36th annual meeting of the Society for Human Reproduction and Embryology, 2020.
9. Balaban B, Isiklar A, Urman B, Gardner DK, Larman MG. Vitrification of human and mouse embryos using the Rapid-i. Fertil Steril. 2010 Sep;94(4): S115.
10. Pinasco M, Hickman T, Russell H, Rashiv B. Oocyte Vitrification Freeze/Thaw Survival Rates Using an Open Versus a Closed System. Fertil Steril. 2012 March;97(3):S18.
11. Machac S, Hubinka V, Larman M, Koudelka M. A novel method for human oocyte vitrification with a closed device using super-cooled air. Fertil Steril. 2013 Oct;100(3):S108.
12. Perez O, Tilley B, Navarrete G, Lay L, Little LM, Gada R, Chantilis S. Oocyte vitrification using a new vitrification medium and a new closed vitrification device. A sibling oocyte study. Fertil Steril. 2018 Sep;110(4):E179-E180.
13. Pujol A, Zamora MJ, Obradors A, Garcia D, Rodriguez A, Vassena R. Comparison of two different oocyte vitrification methods: a prospective, paired study on the same genetic background and stimulation protocol. Hum Reprod. 2019 Jun 4;34(6):989-997.
14. Vitrolife data-on-file 2022.
Support Documents
Documents Language

Package inserts
English
Package insert RapidVit Blast - Multi-language
Document and Ref: REF 26085, version 11

Package inserts
English
Package insert RapidWarm Blast - Multi-language
Document and Ref: REF 26086, version 11

Safety data sheets (SDS)
English
SDS RapidVit Blast
Document and Ref: Document and Ref: Date Revised : 04/16/2025.Revision No : 6. Safety data sheet for RapidVit Blast

Safety data sheets (SDS)
English
SDS RapidWarm Blast
Document and Ref: Document and Ref: Date Revised : 04/16/2025.Revision No : 5. Safety data sheet for RapidWarm Blast

Short protocols
English
Rapid-i™ Vitrification System of blastocysts
Document and Ref: REF 19128. Version 03. Date of issue 220923. This short protocol describes how to vitrify and warm blastocysts using Rapid-i™ vitrification system and RapidVit™/RapidWarm™ Blast.

SSCP
English
SSCP RapidVit Blast & RapidWarm Blast
Document and Ref: REP-3364. Version 7.0. 2025/03/10
Do you have a question about this product?
Contact us
Find your local Vitrolife representative
Contact usMaximise success every step of the way
We provide what you need to secure improved results throughout the IVF journey.